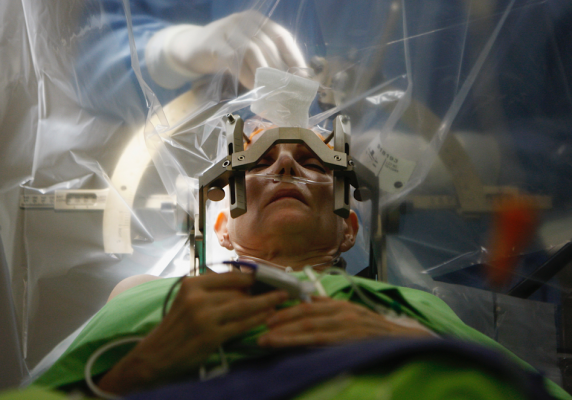
RAD-PD is a collaborative Quality Improvement Research Project, a form of health services effectiveness research that intends to generate a huge amount of information from patients receiving deep brain stimulation surgery for Parkinson’s disease in a systematic goal driven process across multiple institutes. Collection of such a large data set will provide insights into treatment patterns and related outcomes.
What we know about the use of Deep Brain simulation surgery comes from a handful of trials. Designing a trial is important to prove the effectiveness of a treatment. But the same time it is very limited in its scope. Only a few dozen or a few hundred patients are enrolled in a trial at best. If you take all the patients in various randomized deep brain stimulation surgery trials so far together you will hardly reach a number of 1000 patients probably much less than that.
 However nearly 150000 patients have received deep brain stimulation surgery since the approval of this therapy which means that a huge amount of information available in a real clinical care setting has been lost. It’s lost for various reason. it’s lost because not all places collect the information in a searchable manner and not collect the same type of information. And there is no standardized or central way of collecting and sorting such the information.
However nearly 150000 patients have received deep brain stimulation surgery since the approval of this therapy which means that a huge amount of information available in a real clinical care setting has been lost. It’s lost for various reason. it’s lost because not all places collect the information in a searchable manner and not collect the same type of information. And there is no standardized or central way of collecting and sorting such the information.
These days we are realize the importance of practice base research, where information collected during routine clinical care is considered valuable research information.We’re always learning from our patients no matter what we are doing and sometimes the lessons learned in real clinical care are far more useful and appropriate than what we learn from a research setting. Which brings me to this quality improvement project. I think it’s a huge deal. We intend to collect a far larger amount of data from a really big number of patients much bigger than any of the largest DBS trials.
This project is being done in collaboration with Michael J Fox Foundation which is the primary source of funding and has been organized by a group of researchers called Parkinson’s Study Group. The top notch researchers in the field of Parkinson’s disease such are members of this elite research group. The trial intends to involve 10 universities in the first year and then add another 10 universities in its second year. University of Nebraska Medical Center was one of the 40 applicants to be selected as the first tier for this prestigious and highly important trial and we were successful in winning a position in the top 10. The project launch is expected later in summer 2018.

We intend to achieve the following three goals with RAD-PD. 1. Identifying the best practices surrounding DBS therapy. Who are the right patients? What are the factors we need to consider before, during and after surgery. What is the best management? 2. We intend to identify the adverse events and factors that determine those adverse events with the DBS therapy including surgical and post-operative complications, long term device therapy, hospitalizations and even death. 3. Health economics and disparities. We want to understand the economics of healthcare delivery in DBS therapy and what disparities exist among various groups of population. We also want to look at differences and similarities in motor and non-motor outcomes. Treatment costs and quality of life improvement with this therapy.
I have talked to my patients whenever selecting the DBS surgery about the side effects and I tell them about the risk of bleeding in the brain that could lead to death. But we have no way of finding out who among the surgical candidates will be at risk for any particular side effect. Hopefully with this data being collected we will be able to answer that important question.
The data will be collected in coordination with a central data collection site or repository that will coordinate with all the sites to store data in a central location. The data will be accessible by all investigators immediately but in the near future we intend to make the data available to all researchers in Parkinson’s disease. We don’t want data to be locked away as a treasure.
We expect to have a large number of the patient population in this study. Our estimates are that by the end of first year we’ll have more than 150 patients in this registry. By the end of second year, the number will go up to 450 or higher and by the end of the third year we expect to enroll nearly 790 patients into this registry. These are estimates of course and the numbers could be higher or lower than this. But in this five year long project we expect to have more than a thousand number of patients enrolled with providing very useful information in a coordinated manner.
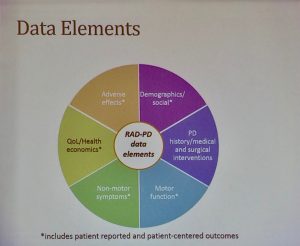 What kind of data will be gather? We will gather all kind of data, Demographics and social outcomes both patient and physician reported, Parkinson’s disease history, medical and surgical interventions, operative procedures and post op complications, effects on motor function and non-motor symptoms, effect on quality of care, cost of surgeries, and healthcare economics.
What kind of data will be gather? We will gather all kind of data, Demographics and social outcomes both patient and physician reported, Parkinson’s disease history, medical and surgical interventions, operative procedures and post op complications, effects on motor function and non-motor symptoms, effect on quality of care, cost of surgeries, and healthcare economics.
The data is being collected as part of the routine ongoing clinical care of the patients. Patients are not being offered DBS for the study specifically but as part of their routine clinical care. A lot of what we are gathering has already been collected at multiple sites in different forms and shapes. The main change that we would be collecting a more uniform data and importantly collecting this data in a uniform fashion in a central secure location where the data can be accessible for all to start seeing trends which are hard to see at smaller scale.
I’m really excited about the study and I hope that we will learn many and I’m sure we will learn many useful lessons from this study over the next 2-3 years. Stay tuned for the results.




